

 Overview
Overview The SurgTech Interbody System is a system of intervertebral body fusion devices. The Posterior Lumbar Device is a structural column in a generally rectangular shape having a rounded nose. Teeth are integral to the inferior and superior surfaces and there is a central cavity to be filled with autograft. The implants are available in an assortment of footprint, height and angulation combinations to accommodate a variety of anatomic requirements.
The SurgTech Interbody System cages are manufactured from PEEK Optima LT1 (poly-ether-ether-ketone) per ASTM F2026 and contain tantalum radiopaque markers per ASTM F560.
 Features
Features  Instruments
Instruments  Indication
Indication
The SurgTech Interbody System is indicated for use with autogenous bone graft in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have had six months of non-operative treatment. These patients may have had a previous non-fusion spinal surgery and may also have up to Grade 1 spondylolisthesis or retrolisthesis at the involved spinal levels. These devices are intended to be used with supplemental fixation which has been cleared for use in the lumbar spine (i.e. a posterior pedicle screw and rod system).
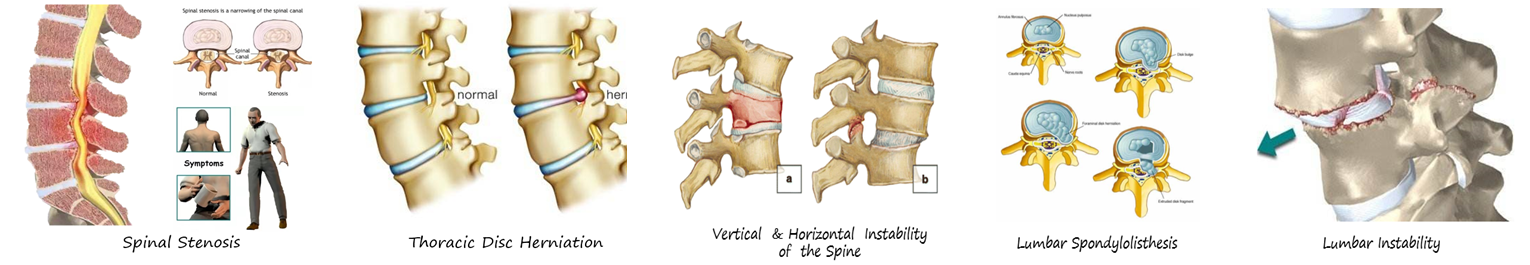
1. Thoracic Disc Herniation (TDH); Spinal Stenosis; Degenerative Intervertebral Instability.
2. Postoperative Lumbar Instability Requiring Simultaneous Posterior Pedicle Screw Fixation.
3. Discogenic Low Back Pain (DLBP) with Limited Anterior Surgery.
4. Patients with Lumbar Spondylolisthesis of Various Causes Requiring Simultaneous Decompression and Reduction and Fixation of the Spinal Canal.
5. Thoracolumbar Tuberculosis.
6. Thoracolumbar Fracture.
7. Congenital Deformity of Spine.
8. Developmental Deformity of Spine.
Contact
Tel: (440) 899-2922 E-mail: chengbao@surgtech-med.com Address: 24600 Center Ridge Road, Suite 195 Westlake OH 44145